| |
What Is GMP?
GMP refers to the Good Manufacturing Practice Regulations promulgated by the US Food and Drug Administration under the authority of the Federal Food, Drug, and Cosmetic Act (See Chapter IV for food, and Chapter V, Subchapters A, B, C, D, and E for drugs and devices.) These regulations, which have the force of law, require that manufacturers, processors, and packagers of drugs, medical devices, some food, and blood take proactive steps to ensure that their products are safe, pure, and effective. GMP regulations require a quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mixups, and errors. This in turn, protects the consumer from purchasing a product which is not effective or even dangerous. Failure of firms to comply with GMP regulations can result in very serious consequences including recall, seizure, fines, and jail time. GMP regulations address issues including recordkeeping, personnel qualifications, sanitation, cleanliness, equipment verification, process validation, and complaint handling. Most GMP requirements are very general and open-ended, allowing each manufacturer to decide individually how to best implement the necessary controls. This provides much flexibility, but also requires that the manufacturer interpret the requirements in a manner which makes sense for each individual business. GMP is also sometimes referred to as "cGMP". The "c" stands for "current," reminding manufacturers that they must employ technologies and systems which are up-to-date in order to comply with the regulation. Systems and equipment used to prevent contamination, mixups, and errors, which may have been "top-of-the-line" 20 years ago, may be less than adequate by today's standards. 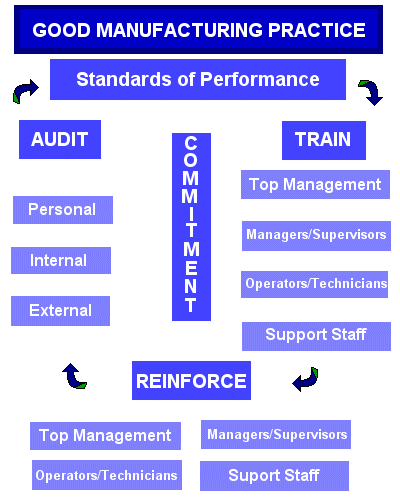 At the GMP Institute, we believe that GMP is a good business tool which will help to refine both compliance and performance at your company. GMP requirements are largely common sense practices which will help your company better itself as it moves toward a quality approach using continuous improvement. The diagram at left illustrates how we approach creating and maintaining a GMP lifestyle in a company. First, set standards of performance. These include GMP regulations and other standards which are necessary for your company. Then, train to those standards. All departments in the company should be trained (to varying degrees) on GMP and other standards. The diagram lists four types of employees which are especially critical to train: top management, managers and supervisors, operators and technicians, and support staff. Because training is such an important part of maintaining a GMP Lifestyle, the GMP Institute focuses heavily on training. We offer many workshops for a variety of types of people. GMP 101 is a great introduction to the concepts of GMP and the reason behind it. Additionally, we offer workshops to help in house trainers perfect the art of training, and to help them learn how to make GMP an interesting topic. We even offer a certification program for trainers. The next step in the GMP Lifestyle is to reinforce what was learned in training. This falls on the managers and supervisors in a plant. Therefore, it is important that managers and supervisors be involved in training, so that they can support it through reinforcement. The same four job categories are listed as being the most critical in promoting and receiving reinforcement. The third stage is to audit to ensure that your efforts have provided adequate controls by auditing. Audits fall in the following three categories: personal, whereby every individual does a self-check to make sure that he/she is complying with all appropriate standards; internal audit, which should be performed by the quality assurance department as required by GMP, and external audits, which can consist of an FDA audit, a consultant checking your compliance status, or you performing a supplier audit. The GMP Institute also offers workshops for auditors needing training. Finally, the results of audits will help you to know if you need to modify your standards of performance. Of course, no procedures should be changed without appropriate change control and approval from quality assurance. The glue that sticks the whole process together is commitment. Commitment to GMP and quality is critical at all levels of the organization, starting with top management. If you foster commitment, use this process, and attend GMP Institute workshops when necessary, you will help you make GMP a Lifestyle, Not Just a Regulation in your company. You will then improve the overall performance of your workforce, as well as your FDA compliance. Peruse the GMP regulations! At the GMP Institute, we believe that GMP is a good business tool which will help to refine both compliance and performance at your company. GMP requirements are largely common sense practices which will help your company better itself as it moves toward a quality approach using continuous improvement. The diagram at left illustrates how we approach creating and maintaining a GMP lifestyle in a company. First, set standards of performance. These include GMP regulations and other standards which are necessary for your company. Then, train to those standards. All departments in the company should be trained (to varying degrees) on GMP and other standards. The diagram lists four types of employees which are especially critical to train: top management, managers and supervisors, operators and technicians, and support staff. Because training is such an important part of maintaining a GMP Lifestyle, the GMP Institute focuses heavily on training. We offer many workshops for a variety of types of people. GMP 101 is a great introduction to the concepts of GMP and the reason behind it. Additionally, we offer workshops to help in house trainers perfect the art of training, and to help them learn how to make GMP an interesting topic. We even offer a certification program for trainers. The next step in the GMP Lifestyle is to reinforce what was learned in training. This falls on the managers and supervisors in a plant. Therefore, it is important that managers and supervisors be involved in training, so that they can support it through reinforcement. The same four job categories are listed as being the most critical in promoting and receiving reinforcement. The third stage is to audit to ensure that your efforts have provided adequate controls by auditing. Audits fall in the following three categories: personal, whereby every individual does a self-check to make sure that he/she is complying with all appropriate standards; internal audit, which should be performed by the quality assurance department as required by GMP, and external audits, which can consist of an FDA audit, a consultant checking your compliance status, or you performing a supplier audit. The GMP Institute also offers workshops for auditors needing training. Finally, the results of audits will help you to know if you need to modify your standards of performance. Of course, no procedures should be changed without appropriate change control and approval from quality assurance. The glue that sticks the whole process together is commitment. Commitment to GMP and quality is critical at all levels of the organization, starting with top management. If you foster commitment, use this process, and attend GMP Institute workshops when necessary, you will help you make GMP a Lifestyle, Not Just a Regulation in your company. You will then improve the overall performance of your workforce, as well as your FDA compliance. Peruse the GMP regulations!
21 CFR Parts 210 and 211 (Drug Industry)
21 CFR Part 820 (Medical Device Industry)
21 CFR Part 110 (Food Industry)
21 CFR Part 606 (Blood Industry)
|
|


 At the GMP Institute, we believe that GMP is a good business tool which will help to refine both compliance and performance at your company. GMP requirements are largely common sense practices which will help your company better itself as it moves toward a quality approach using continuous improvement. The diagram at left illustrates how we approach creating and maintaining a GMP lifestyle in a company. First, set standards of performance. These include GMP regulations and other standards which are necessary for your company. Then, train to those standards. All departments in the company should be trained (to varying degrees) on GMP and other standards. The diagram lists four types of employees which are especially critical to train: top management, managers and supervisors, operators and technicians, and support staff. Because training is such an important part of maintaining a GMP Lifestyle, the GMP Institute focuses heavily on training. We offer many
At the GMP Institute, we believe that GMP is a good business tool which will help to refine both compliance and performance at your company. GMP requirements are largely common sense practices which will help your company better itself as it moves toward a quality approach using continuous improvement. The diagram at left illustrates how we approach creating and maintaining a GMP lifestyle in a company. First, set standards of performance. These include GMP regulations and other standards which are necessary for your company. Then, train to those standards. All departments in the company should be trained (to varying degrees) on GMP and other standards. The diagram lists four types of employees which are especially critical to train: top management, managers and supervisors, operators and technicians, and support staff. Because training is such an important part of maintaining a GMP Lifestyle, the GMP Institute focuses heavily on training. We offer many